de Broglie Wavelength
de Broglie Wavelength: Overview
This topic covers concepts, such as Relation Between de-Broglie Wavelength and Circumference of Bohr's Radius, Relation of Wavelength of Electron with the Accelerating Voltage, Diffraction of Electron, de-Broglie Wavelength, etc.
Important Questions on de Broglie Wavelength
The energy of the electron, in the hydrogen atom, is known to be expressible in the form
sing this expression, which of the following statement is/are true?
(i) Electron in the hydrogen atom cannot have energy of .
(ii) Spacing between the lines (consecutive energy levels) within the given set of the observed hydrogen spectrum decreases as n increases.
For what kinetic energy of a proton, will the associated de Broglie wavelength be 16.5 nm?
For what kinetic energy of a neutron the associated de Broglie wavelength be ?
An particle and a proton are accelerated from rest by the same potential. The ratio of their de Broglie wavelengths would be:
An particle and a proton are accelerated from rest by the same potential. The ratio of their de Broglie wavelengths would be,
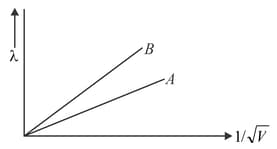
Two lines, A and B, in the plot given below show the variation of de Brogile wavelength, versus , where V is the accelerating potential difference, for two particles carrying the same charge. Which one of two represents a particle of smaller mass?
An -particle and a proton are accelerated from rest through the same potential difference V. Find the ratio of de-Broglie wavelengths associated with them.
An -particle and a proton are accelerated from rest through the same potential difference V. Find the ratio of de-Broglie wavelengths associated with them.
What is the significance of Davisson- Germer experiment?
The Davisson-Germer experiment demonstrated
de Broglie wavelength of an electron is . The energy of the electron is
Planks constant =
Charge of electron=
A neutron has a kinetic energy of Its de-Broglie wavelength is approximately
de Broglie wavelength of an electron having a kinetic energy of is What would be the de Broglie wavelength if it had a kinetic energy of
Calculate the percentage change in the de-Broglie wavelength of the particle, if the kinetic energy of the particle is increased to times its previous value.
Find the ratio of kinetic energy of the electron to that of the energy of photon, if de Broglie wavelength of an electron moving with velocity , is equal to that of a photon.
A constant electric field accelerates a charged particle of mass and charge , which was initially at rest. The rate of change of de-Broglie wavelength of this electron at time ignoring relativistic effects is:
From the following, moving with the same velocity, the one which has the largest wavelength:
The relativistic expression for the wavelength of electrons moving with very high speed is
A particle of charge, and mass, enters a region of a transverse electric field of, with an initial velocity, . The time taken for the change in the de-Broglie wavelength of the charge from the initial value of to is proportional to,
Davisson and Germer experiment was the experimental discovery of :
